Carbon filler improves Lithium-ion battery performance
New research published in Applied Physics Reviews claims to offer a solution to the issue of lithium-ion batteries not being able to provide high power output while simultaneously allowing reversible energy storage.
The solution, presented by scientists with the University of Texas at Austin, Stony Brook University, and the Brookhaven National Laboratory shows how the inclusion of conductive fillers improves battery performance.
In their paper, the researchers say the optimum battery design involves thick electrode structures to enhance energy density. However, such a design suffers from poor lithium-ion transport, a key step in the functioning of these electrodes. Various improvement techniques have been tried, including building vertically aligned channels or creating pores of the proper size to facilitate the transport of lithium ions.
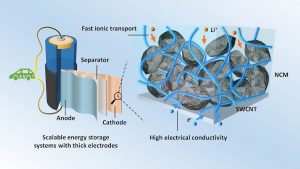
Another approach – they say – involves the use of fillers made of carbon that conduct electricity.
Their study considered three types of fillers: single-walled carbon nanotubes (SWCNTs), graphene nanosheets, and a substance known as Super P, a type of carbon black particles produced during oxidation of petroleum precursors. Super P is the most commonly used conductive filler in lithium-ion batteries.
In their experiments, the fillers were added to a type of electrode material known as NCM that contains nickel, cobalt, and manganese. The investigators examined the resulting composites with scanning electron microscopy. The Super P and NCM particles were found to be arranged in a point-to-point contact mode.
The SWCNTs were, however, wrapped around the NCM particles, forming a conductive coating. In addition, networks of interconnected SWCNTs were observed in the spaces between NCM particles. The graphene nanosheets were also wrapped around the NCM electrode particles but not as uniformly as the SWCNTs were.
The SWCNTs were found to be the best conductive filler for NCM electrodes.
According to the experts, the measured conductivity is consistent with percolation theory and since percolation requires a complete pathway through the filler, a sufficient amount of conductive filler is needed.
Therefore, the team considered various amounts of filler and found that combining NCM electrodes with as little as 0.16% by weight of SWCNT produced good electrical conductivity. Higher amounts of Super P and graphene were required to achieve these same results.
![]()





